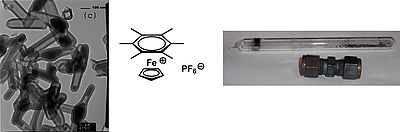
We deal with the presentation and functionalization of new carbon nanomaterials. Depending on the starting material, nanostructures such as graphene, carbon nanotubes, carbon fibers or carbon nanocapsules can be obtained. After functionalization, this opens up a wide range of possible applications, for example in photocatalysis, photoredox synthesis, electrosynthesis, electronic applications or for modifying surface properties. An example of a synthesis of carbon nanocapsules by pyrolysis of an organometallic compound is shown on the left.
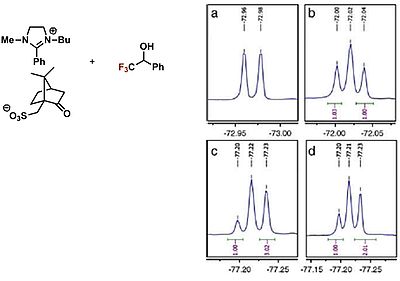
Ionic liquids, which by definition have a melting point of less than 100 °C, are classified as sustainable chemistry. They have a low to zero vapor pressure and can be easily recycled due to their thermal and chemical stability. Chiral ionic liquids can also be used as chiral solvents, shift reagents and in catalysis. We are developing new chiral salts for use in chiral recognition as chiral NMR solvents and in asymmetric catalysis for the visualization of intermediates of biologically active compounds. Another area in which we investigate ionic liquids is the photochemical conversion ofCO2 on TiO2and other semiconductors. The aim is to obtain methane and higher hydrocarbons (such as those found in gasoline), which can serve as energy storage and supply. For this purpose, ionic liquids with dye fragments are synthesized, which are then investigated as photosensitizers in artificial photosynthesis. An example of a chiral ionic liquid as a chiral NMR solvent is shown on the left.
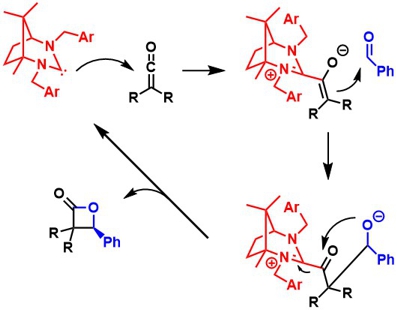
Carbenes are neutral divalent carbon atoms with an electron sextet and can be generated, for example, by deprotonating amidinium salts in situ. In the working group, new chiral amidinium salts are prepared from inexpensive starting materials from the "chiral pool". These are converted into very nucleophilic carbenes, which can be used as organocatalysts for the preparation of biologically active compounds, for example. Furthermore, these carbenes are also used as ligands in various metal-catalyzed asymmetric reactions. Due to the strong s-donor ability, complexes with high activity and high stability can be obtained. An example of an application in an organocatalytic Wynberg reaction is shown on the left.