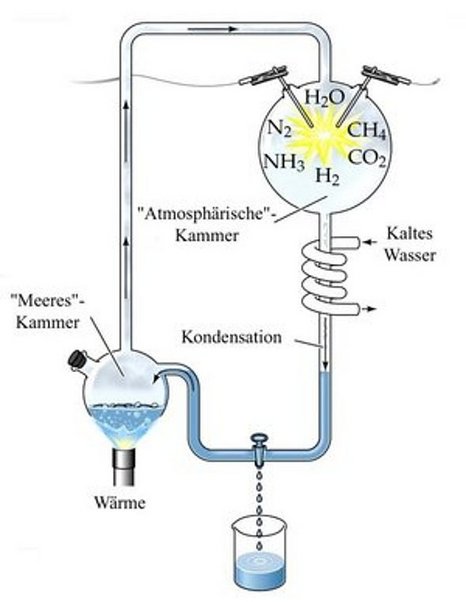
The synthesis of amino acids from inorganic materials according to the historical experiment by Urey and Miller (1953) is of great significance, as it explained the origin of the basic organic building blocks of life from the "primordial soup". This inhospitable atmosphere consisted essentially of ammonia, carbon dioxide, methane and hydrocyanic acid and was discharged by violent thunderstorms.
In our experiment, carbon dioxide and ammonia are converted into electrical discharges of 10000 V AC in a "model thunderstorm". The amino acids already formed after 5 hours are detected by paper or thin-layer chromatography and we try to identify them by means of comparative substances.
At the same time, we can visualize simple amino acids in the laboratory, qualitatively detect sulphur and nitrogen in proteins or proteins in beans, peas or potatoes. Or hydrolyze a protein into its components and then chromatograph it.
Learning objectives: Chemical structure of amino acids and proteins, qualitative detection reactions, paper chromatography and thin-layer chromatography, peptide bond formation and cleavage, amino acid synthesis by nucleophilic substitution, zwitterion form of amino acids, chirality.


