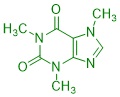
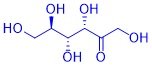
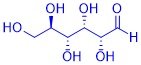
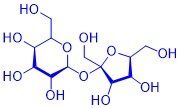
Organic substances can often be perceived through their smell and taste: the taste of vanilla ice cream is just as much based on organic substances as that of a fresh pineapple, a fresh cup of coffee or mown grass. This is why the senses of taste and smell are also referred to as the "chemical senses". How are these substances produced in plants? What is their chemical structure? Why do these compounds smell or taste? What happens when these compounds are chemically modified?
We can now answer many (but by no means all) of these questions. We know the chemical structures of many aroma carriers and ingredients, and can "recreate" some compounds in the laboratory. At the beginning of such a research series, of course, is the isolation from natural material. We want to unlock some of nature's secrets as part of the student internship.
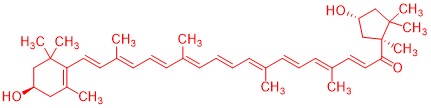
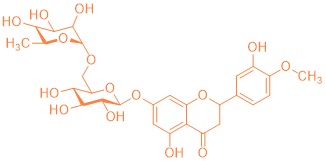
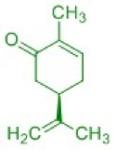
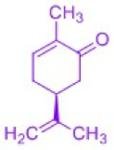
Terpenes play an important biological role as primary and secondary metabolites: plant flavorings, hormones, repellents and pheromones (i.e. also the language of insects) belong to this class of compounds, which, despite the diversity of biological functions, are based on a common organizing principle of their molecular structure (Ruzicka rule). In this subproject, terpenes are isolated from plant material by steam distillation and then purified using chromatographic methods. The related architecture of these isolated molecules requires the study of biosynthesis. The chirality can even be detected by the characteristic odor of the isolated essential oil: Proof of the different biological effects of mirror-image molecules, e.g. using